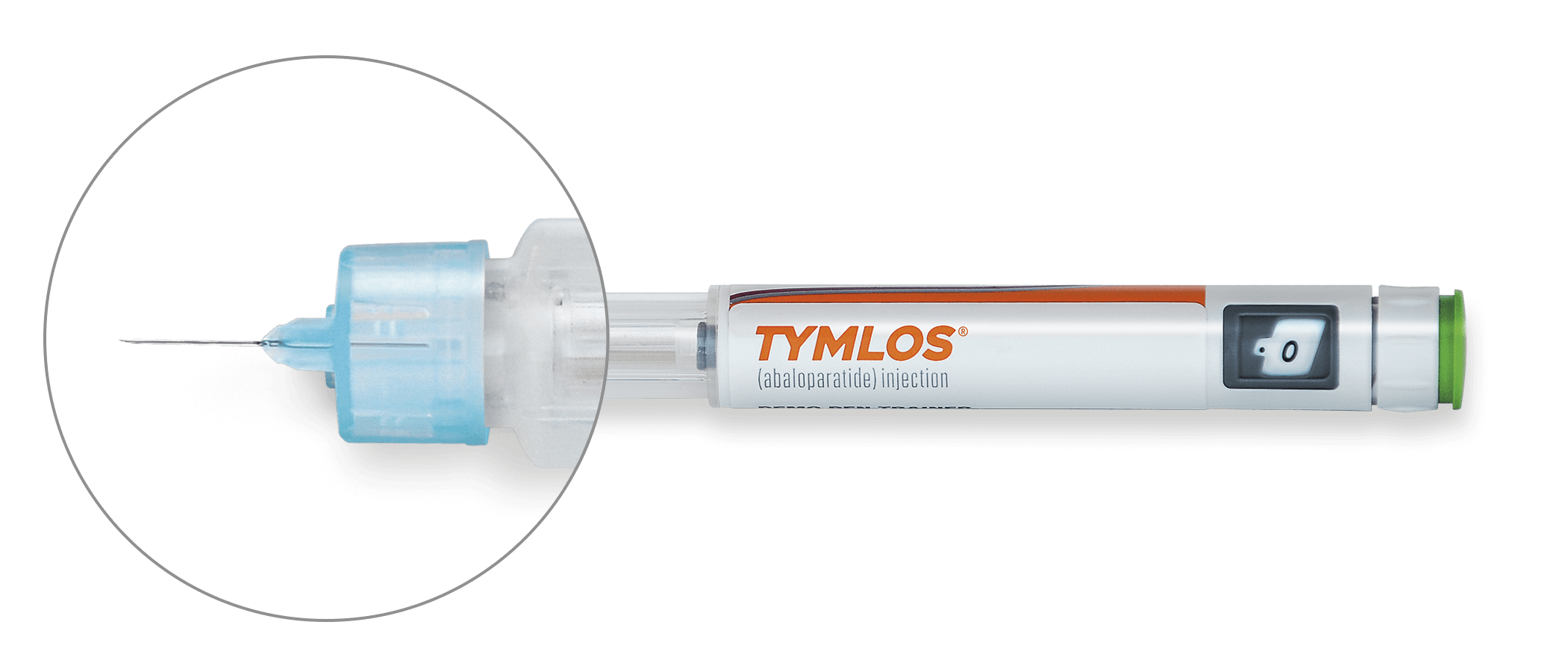
IMPORTANT SAFETY INFORMATION
Contraindications: TYMLOS is contraindicated in patients with a history of systemic hypersensitivity to abaloparatide or to any component of the product formulation. Reactions have included anaphylaxis, dyspnea, and urticaria.
Risk of Osteosarcoma: It is unknown whether TYMLOS will cause osteosarcoma in humans. Osteosarcoma has been reported in patients treated with a PTH-analog in the post marketing setting; however, an increased risk of osteosarcoma has not been observed in observational studies in humans. There are limited data assessing the risk of osteosarcoma beyond 2 years of TYMLOS use. Avoid use of TYMLOS for patients at an increased baseline risk for osteosarcoma including patients with open epiphysis (pediatric and young adult patients); metabolic bone diseases other than osteoporosis, including Paget’s disease of the bone; bone metastases or a history of skeletal malignancies; prior external beam or implant radiation therapy involving the skeleton; or hereditary disorders predisposing to osteosarcoma.
Orthostatic Hypotension: Orthostatic hypotension may occur with TYMLOS, typically within 4 hours of injection. Associated symptoms may include dizziness, palpitations, tachycardia, or nausea, and may resolve by having the patient lie down. For the first several doses, TYMLOS should be administered where the patient can sit or lie down if necessary.
Hypercalcemia: TYMLOS may cause hypercalcemia. TYMLOS is not recommended in patients with pre-existing hypercalcemia or in patients who have an underlying hypercalcemic disorder, such as primary hyperparathyroidism, because of the possibility of exacerbating hypercalcemia.
Hypercalciuria and Urolithiasis: TYMLOS may cause hypercalciuria. It is unknown whether TYMLOS may exacerbate urolithiasis in patients with active or a history of urolithiasis. If active urolithiasis or pre-existing hypercalciuria is suspected, measurement of urinary calcium excretion should be considered.
Pregnancy and Lactation: TYMLOS is not indicated for use in females of reproductive potential.
Adverse Reactions:
- The most common adverse reactions (incidence ≥2%) reported with TYMLOS in postmenopausal women with osteoporosis are hypercalciuria (11%), dizziness (10%), nausea (8%), headache (8%), palpitations (5%), fatigue (3%), upper abdominal pain (3%), and vertigo (2%).
- The most common adverse reactions (incidence ≥2%) reported with TYMLOS in men with osteoporosis are injection site erythema (13%), dizziness (9%), arthralgia (7%), injection site swelling (7%), injection site pain (6%), contusion (3%), abdominal distention (3%), diarrhea (3%), nausea (3%), abdominal pain (2%), and bone pain (2%).
INDICATIONS AND USAGE
TYMLOS is indicated for the:
- treatment of postmenopausal women with osteoporosis at high risk for fracture (defined as a history of osteoporotic fracture or multiple risk factors for fracture), or patients who have failed or are intolerant to other available osteoporosis therapy. In postmenopausal women with osteoporosis, TYMLOS reduces the risk of vertebral fractures and nonvertebral fractures.
- treatment to increase bone density in men with osteoporosis at high risk for fracture (defined as a history of osteoporotic fracture or multiple risk factors for fracture), or patients who have failed or are intolerant to other available osteoporosis therapy.
References: 1. TYMLOS. Prescribing information. Radius Health, Inc. 2. Dempster DW, Zhou H, Rao SD, et al. Early effects of abaloparatide on bone formation and resorption indices in postmenopausal women with osteoporosis. J Bone Miner Res. 2021;36(4):644-653. 3. Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives.J Clin Endocrinol Metab. 2012;97(2):311-325. 4. Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11(4):219-227. 5. Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722-733. Published correction appears in JAMA. 2017;317(4):442. 6. Tay D, Cremers S, Bilezikian JP. Optimal dosing and delivery of parathyroid hormone and its analogues for osteoporosis and hypoparathyroidism—translating the pharmacology. Br J Clin Pharmacol. 2018;84(2):252-267. 7. Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016;157(1):141-149. 8. Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda). 2016;31(3):233-245. 9. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis–2020 update. Endocr Pract. 2020;26(suppl 1):1-46.