

When it's time to rebuild bone, it's time for TYMLOS.1
TYMLOS rebuilds a foundation of new bone and provides clinically meaningful fracture risk reduction.1
TYMLOS efficacy was demonstrated in the ACTIVE trial.1,2
IN POSTMENOPAUSAL WOMEN
Study design: A randomized, multicenter, double-blind, placebo- and active-controlled clinical study in postmenopausal women with osteoporosis (N=2,463) aged 49 to 86 years (mean age 69 years) who were randomized to receive TYMLOS 80 mcg (n=824), placebo (n=821), or teriparatide 20 mcg (n=818) subcutaneously once daily for 18 months to assess efficacy and safety of abaloparatide injection.1,2
Primary endpoint: Incidence of new vertebral fracture at 18 months (TYMLOS vs placebo).*1
Secondary endpoints:
- Incidence of nonvertebral fractures at 18 months (TYMLOS vs placebo and TYMLOS vs teriparatide)†1,2
- BMD change from baseline at the lumbar spine, total hip, and femoral neck at 18 months (TYMLOS vs placebo; comparisons at 6 and 12 months are exploratory)‡1,2
- BMD change from baseline at the lumbar spine, total hip, and femoral neck, comparing teriparatide and TYMLOS at 6 months (comparisons at 12 and 18 months are exploratory)‡2
This study was not designed to provide head-to-head comparative efficacy data and cannot be interpreted as evidence of superiority or noninferiority to teriparatide.
- *
Modified ITT population, which includes patients who had both pretreatment and posttreatment spine radiographs.1
- †
Nonvertebral fractures were measured using the ITT population and excluded fractures of the sternum, patella, toes, fingers, skull, and face, and those associated with high trauma.1
- ‡
Results reported in the ITT population, which included patients randomized in the efficacy study; last observation carried forward.1,2
BMD=bone mineral density; ITT=intent-to-treat.


TYMLOS helps protect patients against fracture while on treatment.1
TYMLOS significantly reduces vertebral and nonvertebral fractures.1
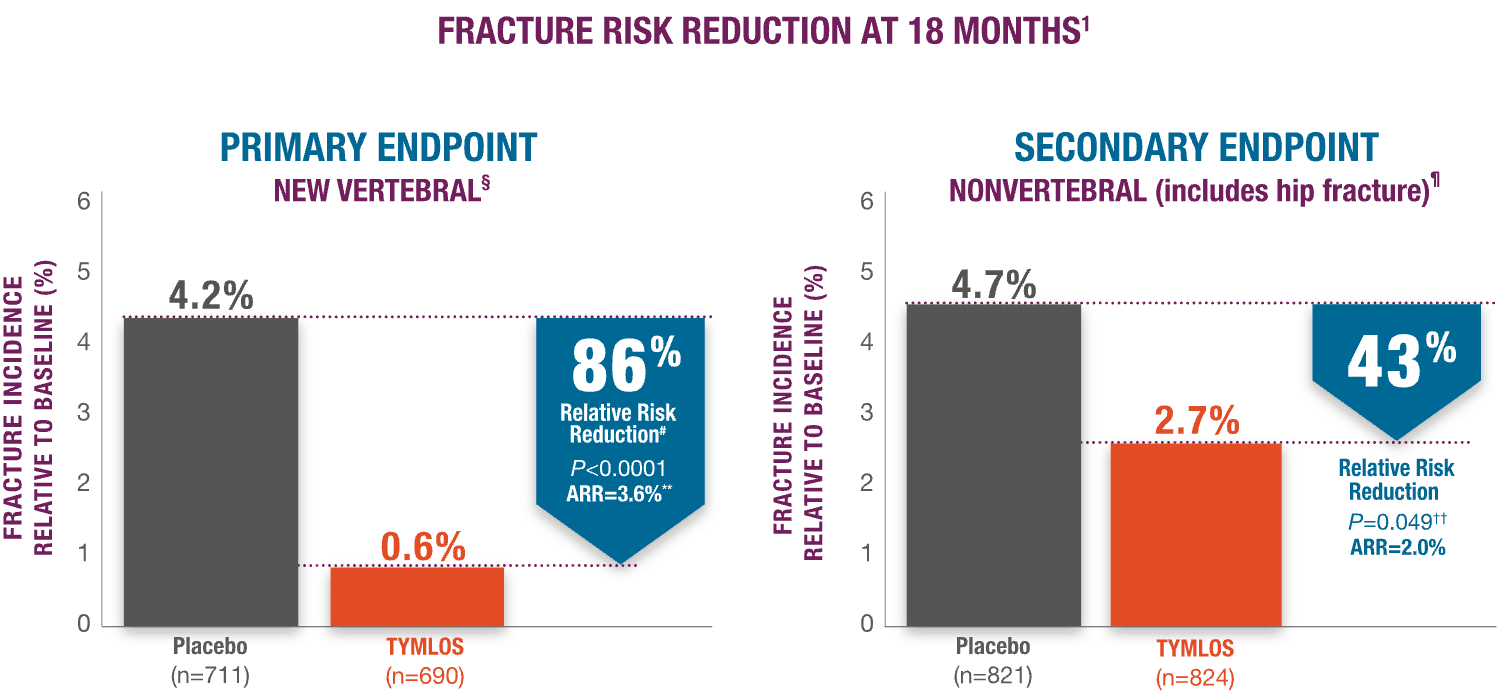

§Modified ITT population, which includes patients who had both pretreatment and posttreatment spine radiographs.
¶95% CI: 61, 95.
#95% CI: 2.1, 5.4.

§Modified ITT population, which includes patients who had both pretreatment and posttreatment spine radiographs.
¶Nonvertebral fractures were measured using the ITT population at 19 months (the entire observational period included 18 months of treatment plus 1 month of follow-up). Nonvertebral fractures excluded fractures of the sternum, patella, toes, fingers, skull, and face, and those associated with high trauma.
#95% CI: 61, 95.
**95% CI: 2.1, 5.4.
††P value based on the log-rank test.
**Nonvertebral fractures were measured using the ITT population at 19 months (the entire observational period included 18 months of treatment plus 1 month of follow-up). Nonvertebral fractures excluded fractures of the sternum, patella, toes, fingers, skull, and face, and those associated with high trauma.
††P value based on the log-rank test.
TYMLOS helps patients build up significant BMD gains vs placebo and protects them against bone loss while on treatment.1-3
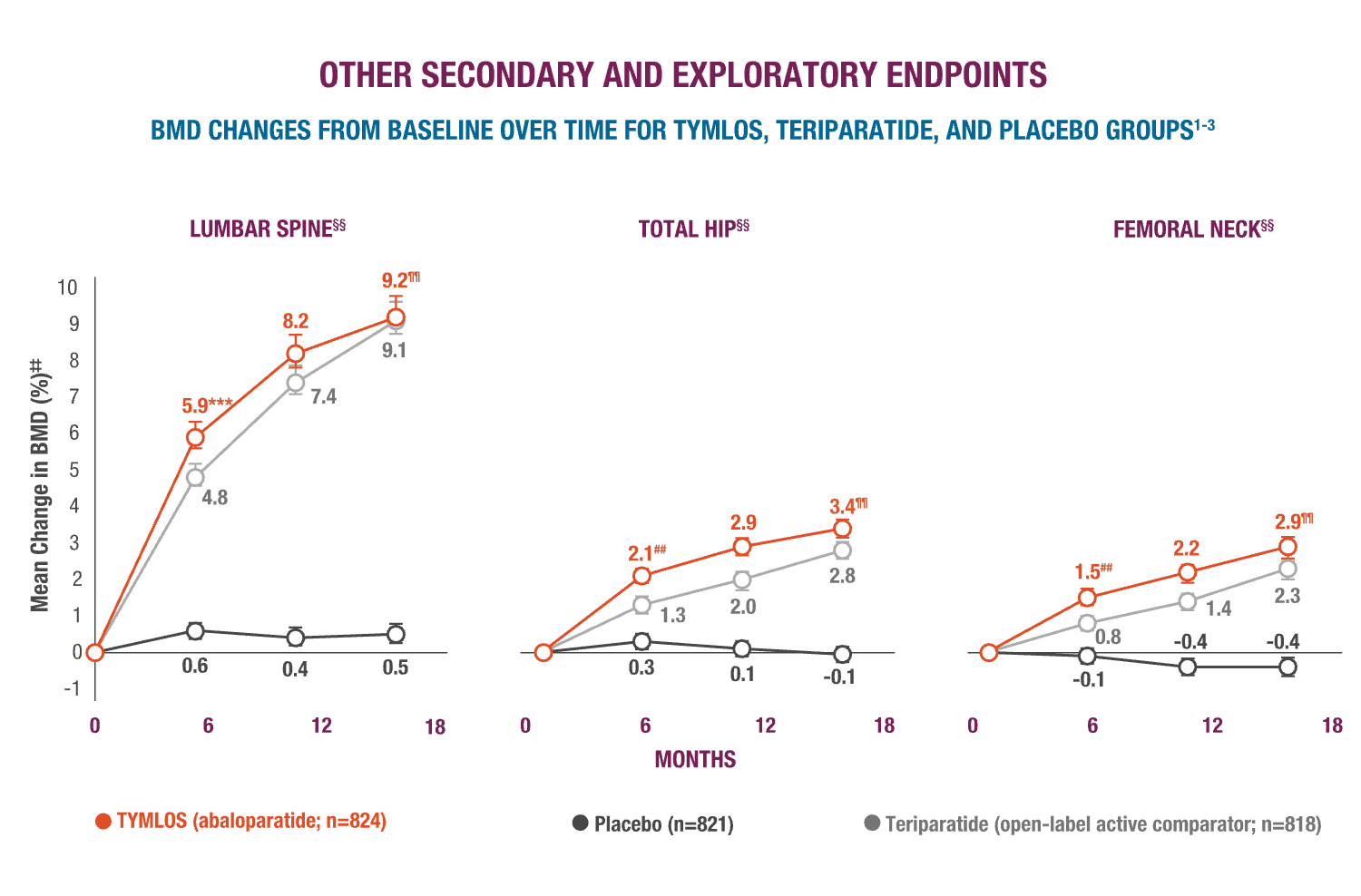
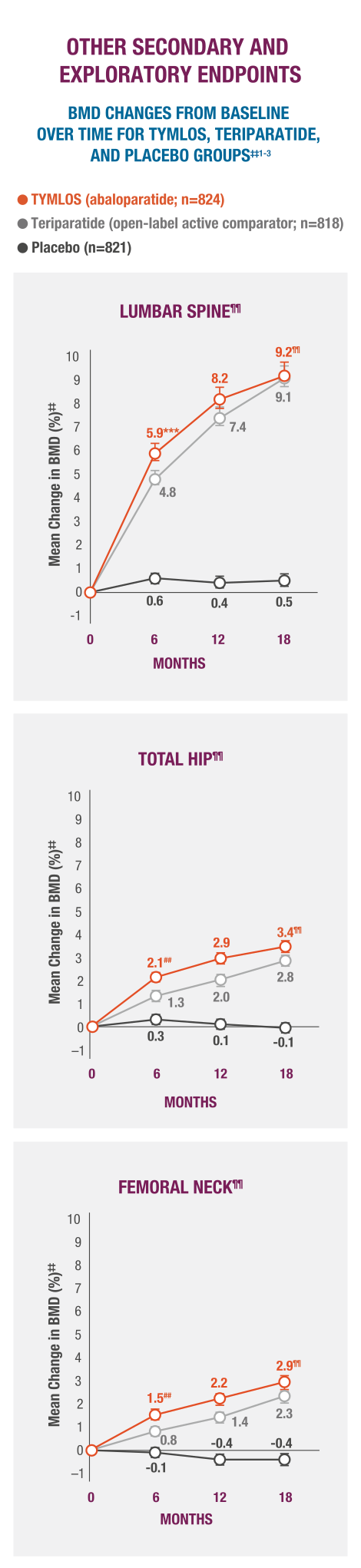
‡‡Results reported in the ITT population, which included patients randomized in the efficacy study; last observation carried forward.1,2
§§Error bars indicate 95% confidence intervals.2,3
¶¶P >0.0001 vs placebo (secondary endpoint).3
##P >0.0001 vs teriparatide.3
***BMD changes at lumbar spine vs teriparatide at 6 months was a forced exploratory endpoint due to hierarchical study design.
ARR=absolute risk reduction;BMD=bone mineral density; CI=confidence interval;ITT=intent-to-treat.
‡‡Results reported in the ITT population, which included patients randomized in the efficacy study; last observation carried forward.1,2
§§As a secondary endpoint, TYMLOS was also compared to teriparatide at 6 months.2,3
¶¶95% CI for TYMLOS (lumbar spine: 8.7, 9.7;total hip: 3.2, 3.7;femoral neck: 2.6, 3.2), for teriparatide(lumbar spine: 8.7, 9.5;total hip: 2.6, 3.0;femoral neck: 2.0, 2.5), and for placebo (lumbar spine: 0.2, 0.7;total hip: -0.3, 0.1;femoral neck: -0.7, -0.2). 3
##P >0.0001 vs placebo (secondary endpoint).3
***BMD changes at lumbar spine vs teriparatide at 6 months was a forced exploratory endpoint due to hierarchical study design.
ARR=absolute risk reduction; BMD=bone mineral density; CI=confidence interval; ITT=intent-to-treat.
TYMLOS has a well-established safety profile.1,2,4,5
Adverse reactions were evaluated in postmenopausal women with osteoporosis.†††1,2

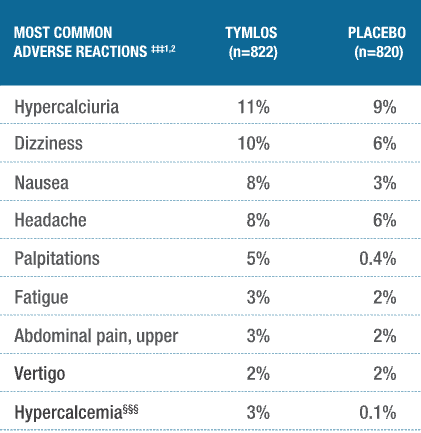
†††The safety analysis included an open-label, active comparative arm with teriparatide (n=818). This study was not designed to provide head-to-head comparative safety data and cannot be interpreted as evidence of superiority or noninferiority to teriparatide.2
‡‡‡Adverse reactions reported in ≥2% of TYMLOS-treated patients.1
§§§Hypercalcemia was a prespecified safety endpoint, defined as albumin-corrected serum calcium of at least 10.7 mg/dL (2.67 mmol/L) at any time point.1,2
There were no discernible differences in serious
adverse events (SAEs) between treatment groups.1
INCIDENCE OF SAEs
TYMLOS:10% vs PLACEBO: 11%
A majority of patients remained on treatment
with TYMLOS.1
DISCONTINUATION RATES
TYMLOS:10% vs PLACEBO: 6%
ACTIVE trial participants included patients with:

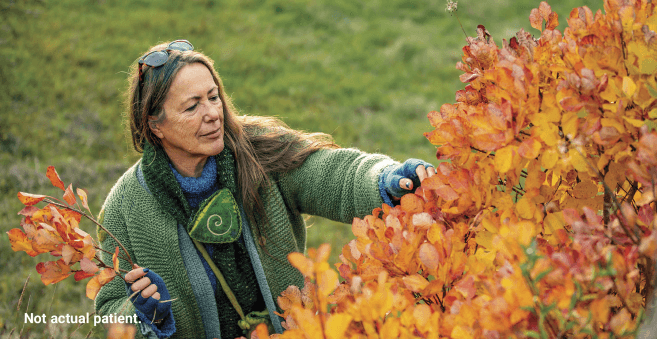
Efficacy was maintained with follow-on therapy in the ACTIVExtend trial.1,4,5
ACTIVExtend—long-term evaluation of TYMLOS efficacy followed with open-label alendronate.1
The extension study evaluated if the fracture risk reductions and increases in BMD achieved with TYMLOS could be maintained with follow-on therapy with alendronate.1,4,5
IN POSTMENOPAUSAL WOMEN
Extension study design: A 24-month, open-label, follow-up study of postmenopausal women who completed the 18-month ACTIVE trial and enrolled in the extension study where they transitioned to alendronate 70 mg weekly as follow-on maintenance therapy from either TYMLOS 80 mcg (n=558) or placebo (n=581).1
Primary endpoint: Percent of patients with ≥1 new vertebral fracture from ACTIVE trial baseline through 6 months of alendronate treatment (Month 25).¶¶¶1
Secondary endpoints:
- Percent of patients with ≥1 new nonvertebral fracture from ACTIVE trial baseline through 6 months of alendronate treatment (Month 25)###1,4
- Mean percentage change in BMD at lumbar spine, total hip, and femoral neck from ACTIVE trial baseline through Month 25###1,4
Exploratory endpoints:
- Percent of patients with ≥1 new vertebral§§§ and nonvertebral fracture at the end of the ACTIVExtend trial (Month 43) §§§
- Mean percentage change in BMD at lumbar spine, total hip, and femoral neck from ACTIVE trial baseline through Month 43###5
¶¶¶Results reported in the modified ITT population, which included patients who had both pretreatment and posttreatment spine radiographs.1
###Results reported in the ITT population, which included patients randomized in the efficacy study. Nonvertebral fractures excluded fractures of the sternum, patella, toes, fingers, skull, and face, and those associated with high trauma.1,4,5
BMD=bone mineral density; ITT=intent-to-treat.

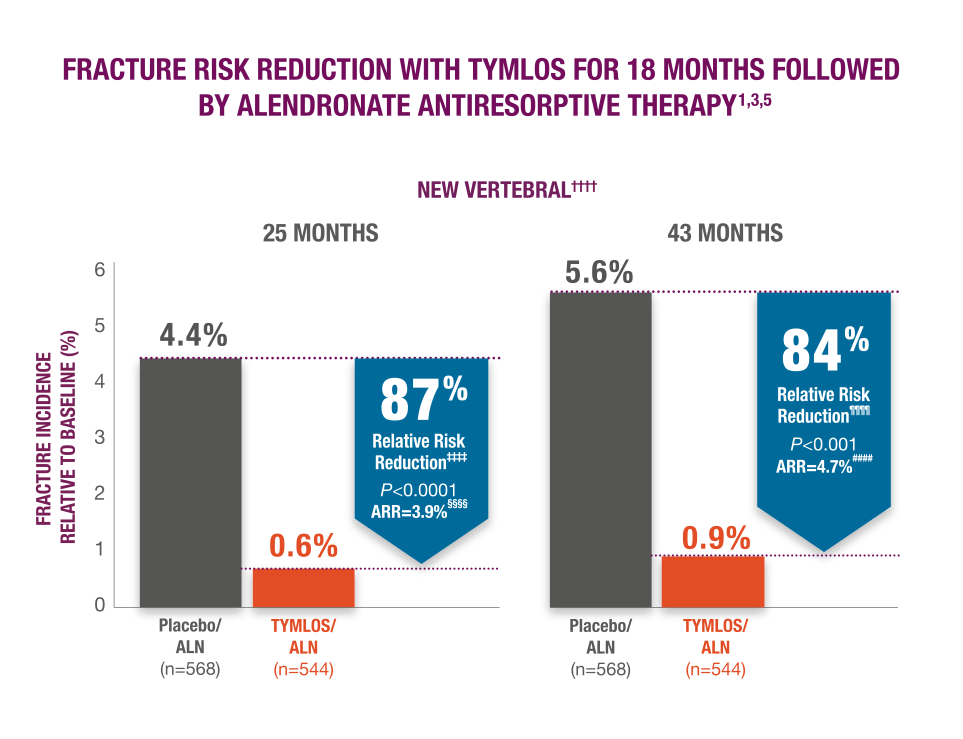
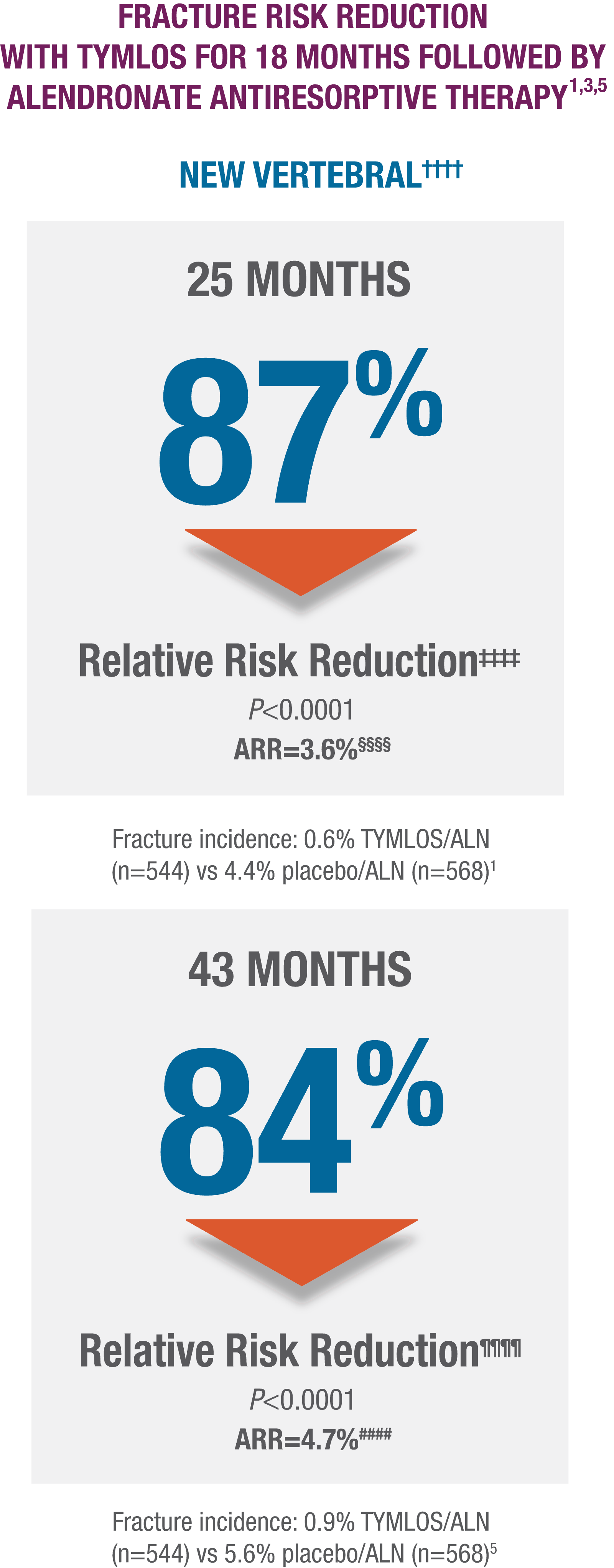
TYMLOS fracture risk reduction was preserved with follow-on therapy.1,4,5
Sustained protection against vertebral fracture was demonstrated for an additional 2 years in the ACTIVExtend trial.****1,4,5
- TYMLOS or placebo for 18 months, followed by 1 month of no treatment, then up to 24 months of open-label alendronate.1
****TYMLOS or placebo for 18 months, followed by 1 month of no treatment, then up to 24 months of open-label alendronate.1
††††Results reported in the modified ITT population, which included patients who had both pretreatment and posttreatment spine radiographs.1,5
‡‡‡‡95% CI: 59, 96.1
§§§§95% CI: 2.1, 5.9. 1
¶¶¶¶95% CI: 58, 94.3
####95% CI: 2.69, 6.99.3
BMD gains with TYMLOS were maintained with follow-on therapy.3
Patients significantly increased BMD with TYMLOS and maintained it after transitioning to alendronate antiresorptive therapy.1,4,5
In a 25-month analysis of the secondary endpoint,***** significant increases in BMD achieved with TYMLOS at 18 months were maintained after transitioning to 6 months of follow-on therapy with alendronate (TYMLOS vs placebo; P>0.001 at all sites)1,4:
- Lumbar spine—12.8% vs 3.5%; treatment difference: 9.3%
- Total hip—5.5% vs 1.4%; treatment difference: 4.1% new vertebral fracture incidence
- Femoral neck—4.5% vs 0.5%; treatment difference: 4.1%
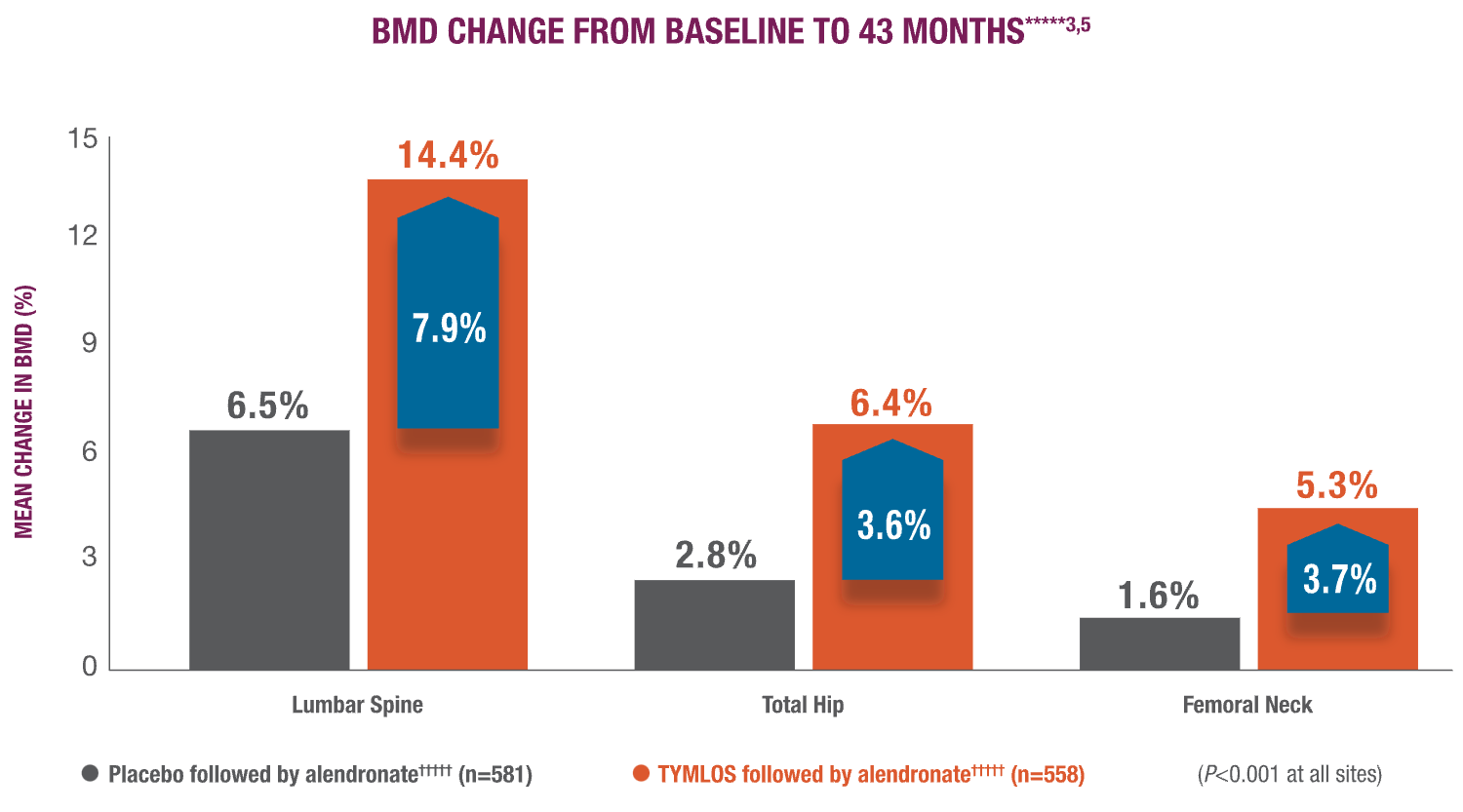
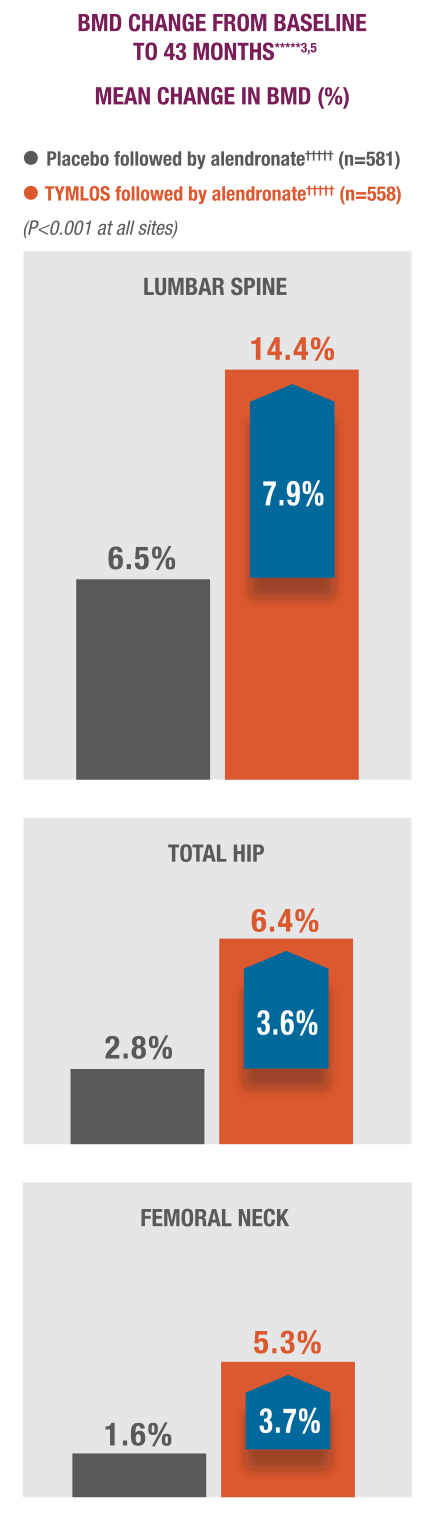
*****TYMLOS or placebo for 18 months, followed by 1 month of no treatment, then up to 24 months of open-label alendronate. The primary and secondary efficacy endpoints for the extension study were at 25 months. The 43-month data are exploratory endpoints. Results reported in the ITT population, which included patients enrolled in the extension study; mean percentage change in BMD was the last observation carried forward.1,3-5
†††††18 months of TYMLOS or placebo to 24 months of open-label alendronate.1
In the ACTIVExtend trial, the overall incidence of adverse events, including severe and SAEs, during the alendronate treatment period was similar for both study groups and was consistent with the recognized profile of alendronate.1,4,5
ALN=alendronate; ARR=absolute risk reduction; BMD=bone mineral density; CI=confidence interval; ITT=intent-to-treat; SAEs=serious adverse events.

Approved for men with osteoporosis at high risk for fracture.1
TYMLOS rebuilds a foundation of new bone.1
Efficacy was established in men with the ATOM trial.1,7
ATOM evaluated the efficacy and safety of TYMLOS in men.1,7
IN MEN
Study design: A Phase 3 randomized, double-blind, placebo-controlled study in men with osteoporosis (N=228) aged 42 to 85 years who were randomized to receive TYMLOS 80 mcg (n=149) or placebo (n=79) subcutaneously once daily for 12 months to assess efficacy and safety of abaloparatide injection.1
Primary endpoint: Change in lumbar spine BMD at 12 months compared with placebo.1
Secondary endpoints:
- Percent change from baseline in total hip and femoral neck BMD at 12 months1,7
- Percent change from baseline in BMD at 3 and 6 months for lumbar spine, total hip, and femoral neck7
TYMLOS quickly and significantly increased BMD in vertebral and nonvertebral bone.1,7
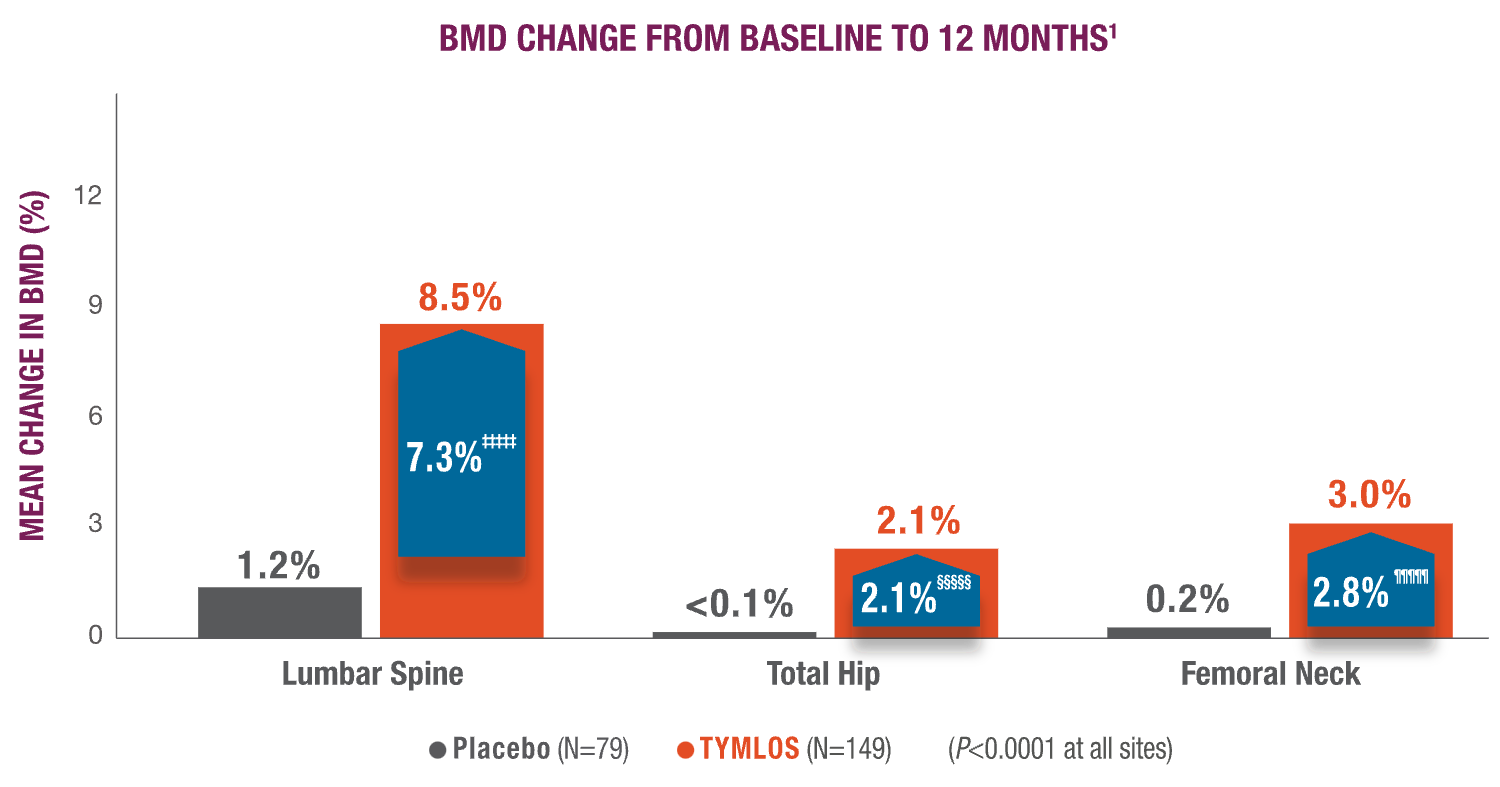
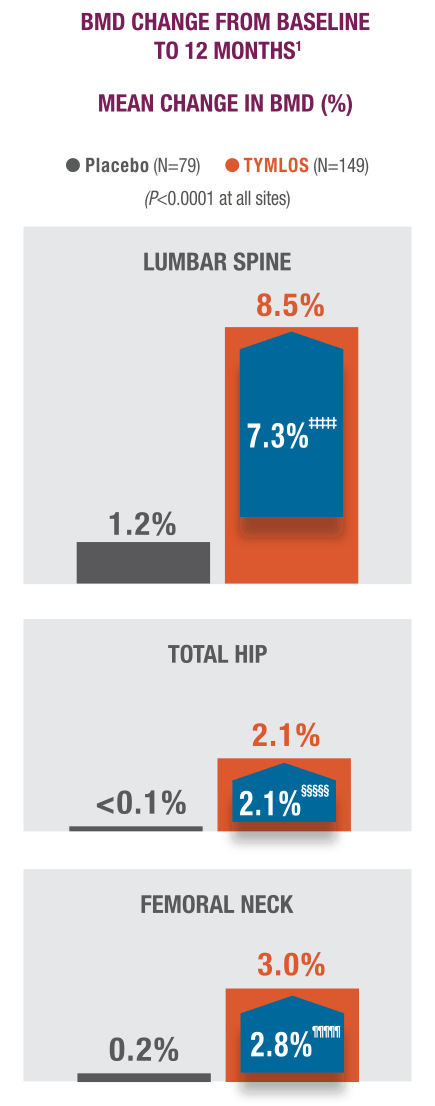
‡‡‡‡‡ 99% CI: 5.1, 9.6.11
§§§§§99% CI: 1.0, 3.2.1
¶¶¶¶¶99% CI: 1.4, 4.2.1
Significantly higher BMD gains were observed at 3 and 6 months with TYMLOS compared to placebo.7
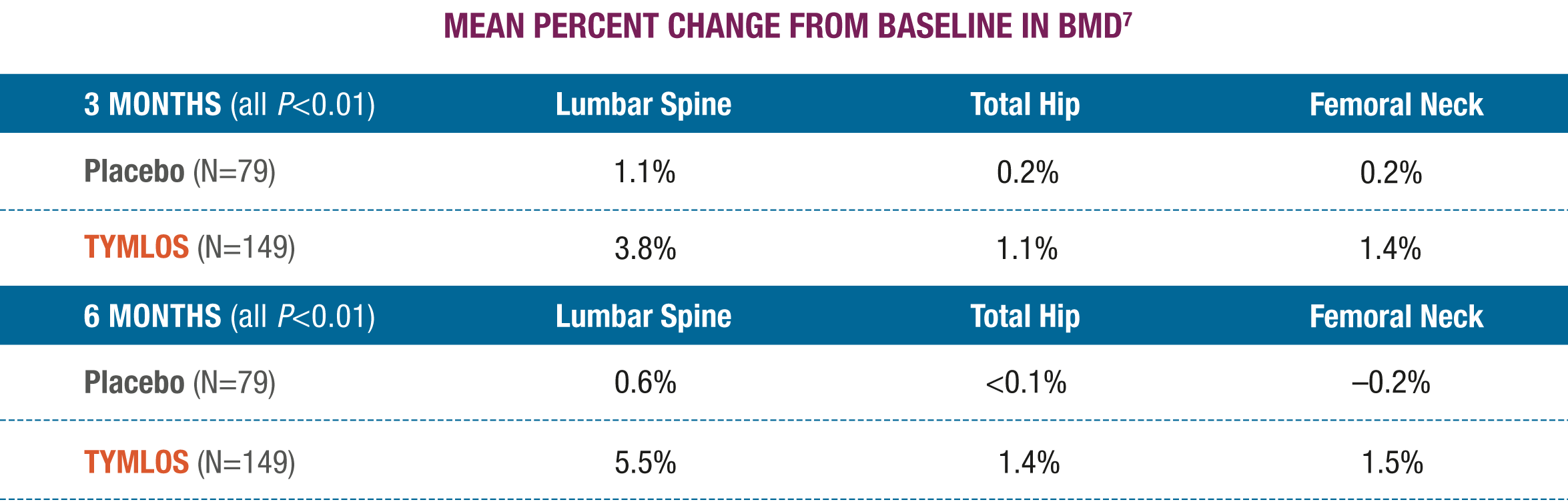
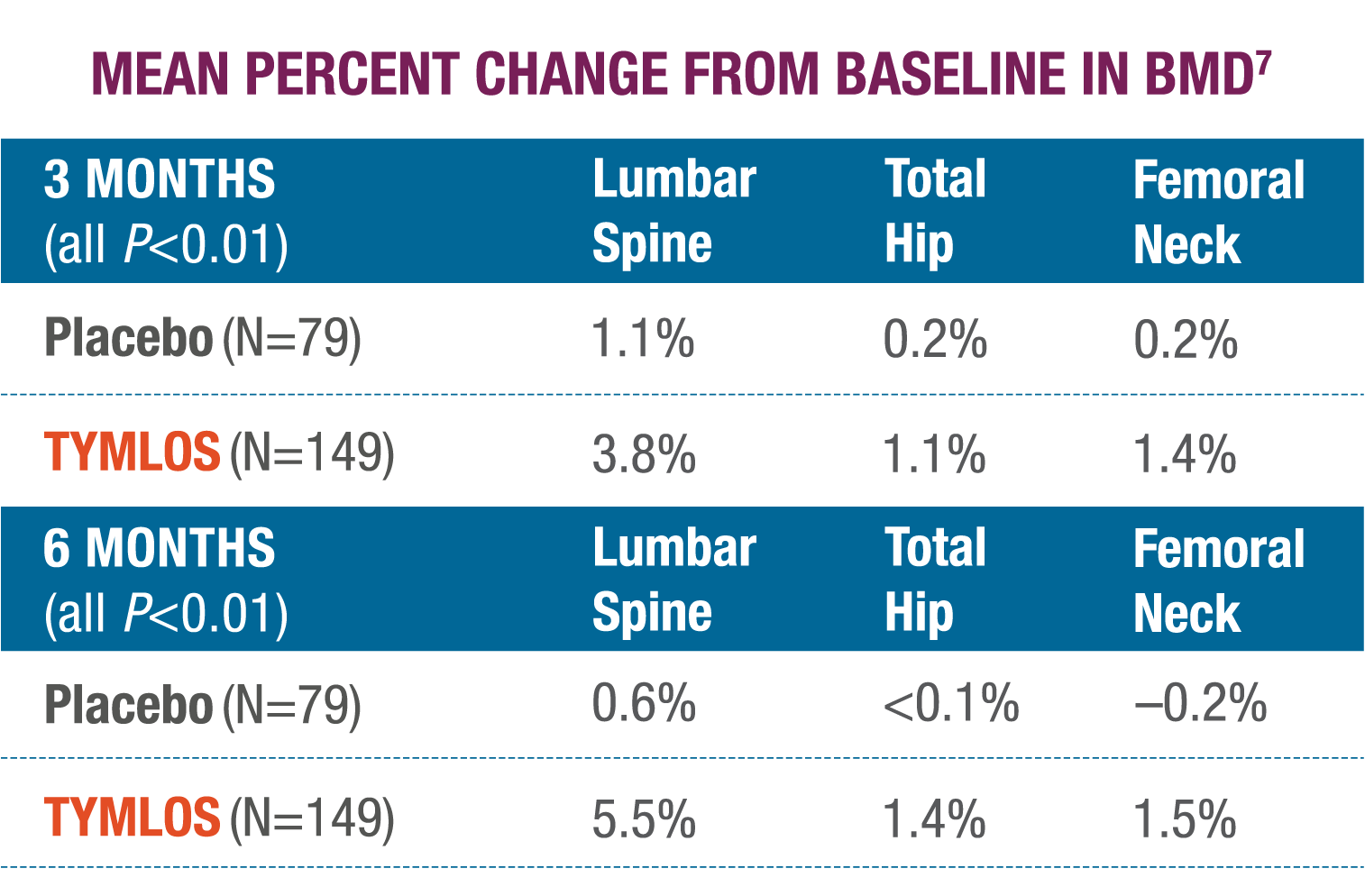
There was no evidence of differences in the effects of TYMLOS on BMD at Month 12 across subgroups defined by age, race, ethnicity, geographic region, presence or absence of prior fracture, and BMD at baseline.
TYMLOS showed a consistent safety profile in the ATOM trial.1
The safety profile for men is consistent with the known safety profile in postmenopausal women with osteoporosis.1

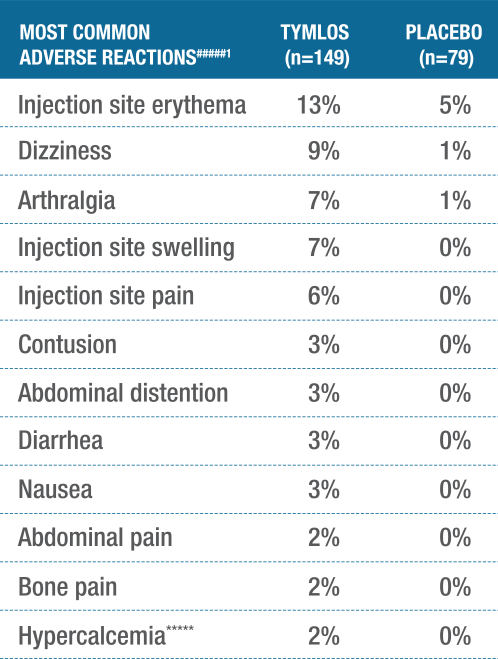
#####Adverse reactions reported in ≥2% of TYMLOS-treated patients.1
******Hypercalcemia was defined as albumin-corrected serum calcium of at least 10.8 mg/dL (2.67 mmol/L) at any time point.1
BMD=bone mineral density; CI=confidence interval.
There were no discernible differences in serious
adverse events (SAEs) between treatment groups.1
INCIDENCE OF SAEs
TYMLOS: 5.4% vs PLACEBO: 5.1%
A majority of patients remained on treatment
with TYMLOS.1
DISCONTINUATION RATES
TYMLOS: 6.7% vs PLACEBO: 5.1%
BMD=bone mineral density; CI=confidence interval.
THE TYMLOS PEN
TYMLOS is self-administered daily using an injection pen.
NEED SUPPORT OR SAMPLES?
Let us know if you want to request samples or a visit from a TYMLOS representative or field reimbursement manager.




