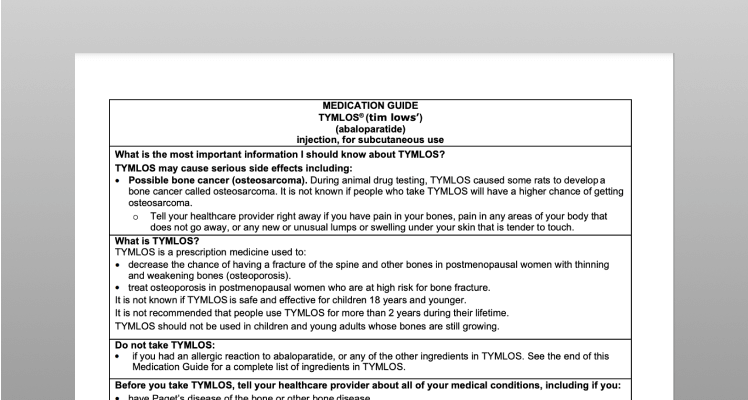
Resources and support
for patients prescribed TYMLOS.
TYMLOS patient support—there
when you and your patients need it.


Not actual patients.
Prescribing resources and downloads.
Patient Enrollment Form
Send your prescription to the Support Center by:
E-prescribing TYMLOS through your EHR system to "Careform Pharmacy" (NPI:1043762750).
Be sure to include the ICD-10 diagnosis code with your e-prescription.
Or
Downloading an enrollment form below, ensuring your patient has signed it, then faxing the completed form, along with a copy of the insurance card (medical and pharmacy), to the Support Center at 1-800-910-4610.
Download (opens in a new tab)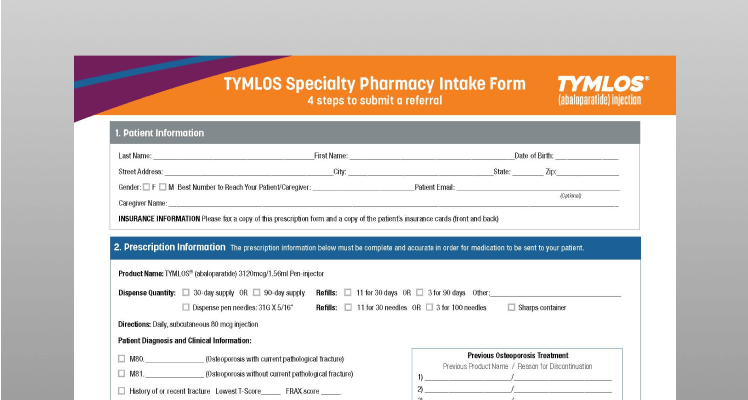
TYMLOS Specialty Pharmacy Intake Form
Complete this form and fax it directly to a specialty pharmacy that dispenses TYMLOS.
Download (opens in a new tab)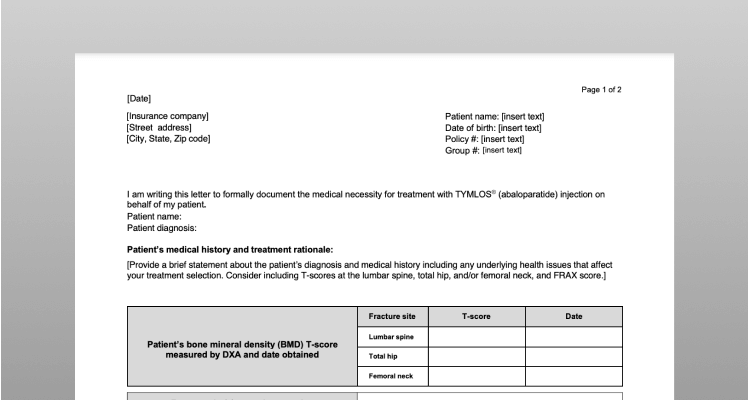
Sample of Prior Authorization (PA) Cover Letter
Some health plans require a PA request form in order to satisfy their policy requirements to obtain TYMLOS coverage. Use this sample letter as a template to help you get started.
Download (opens in a new tab)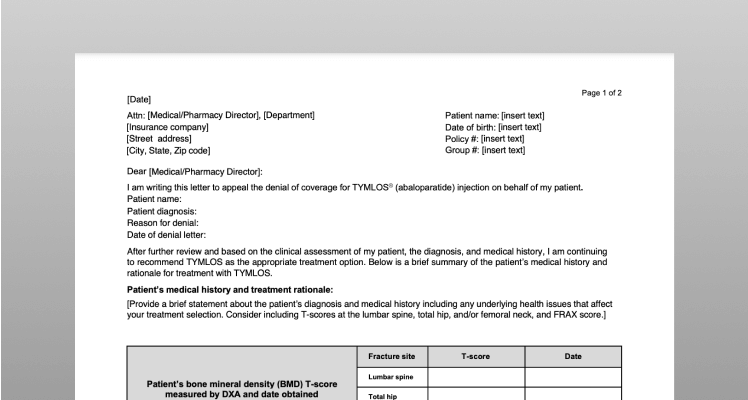
Sample Letter of Appeal
If a health plan denies TYMLOS coverage, they may require a letter of appeal along with a letter of medical necessity in order to reconsider the decision. This template can help you write your own letter of appeal.
Download (opens in a new tab)
Sample Letter of Medical Necessity
Some health plans require a letter of medical necessity in addition to a letter of appeal that may help justify the clinical rationale for covering TYMLOS. Use this template to write a letter of medical necessity.
Download (opens in a new tab)Access and affordability.

From information about insurance coverage to potential savings on treatment, the Radius Support team is available to assist you and your patients.
Call Monday through Friday, 8 am to 7 pm ET, at 1-866-896-5674.
1-866-896-5674
Savings Card
Eligible,* commercially insured patients can pay as little as $0 a month for their TYMLOS prescription. There is an annual cap on the amount of assistance patients can receive over a 1-year period.1
* This offer is not valid for patients using Medicare, Medicaid, or other government-funded programs to pay for their medications. See back of Savings Card for all eligibility requirements, rules, and restrictions. Savings Card is available for patients to download at TYMLOS.com. Limited to one offer per person.1

TYMLOS Specialty Pharmacy (SP) Network List
See a list of non-integrated delivery network SPs that are able to dispense TYMLOS. In addition, SPs affiliated with an integrated delivery network are able to dispense TYMLOS.
Specialty Pharmacy information.
Specialty Pharmacy (SP) information.
Once a prescription has been received, the Support Center will conduct an insurance verification to confirm coverage for TYMLOS, obtain estimated out-of-pocket costs, determine if a prior authorization (PA) is needed, and identify an in-network SP.
Should a PA be required, the SP may reach out to your office for additional information or to submit the PA to the health plan. Prescriptions will be triaged to the patient’s in-network SP whenever possible. The Support Center will provide the pharmacy with the savings card information for eligible patients.
TYMLOS is covered by most commercial and Medicare plans. Individual out-of-pocket costs will vary.1

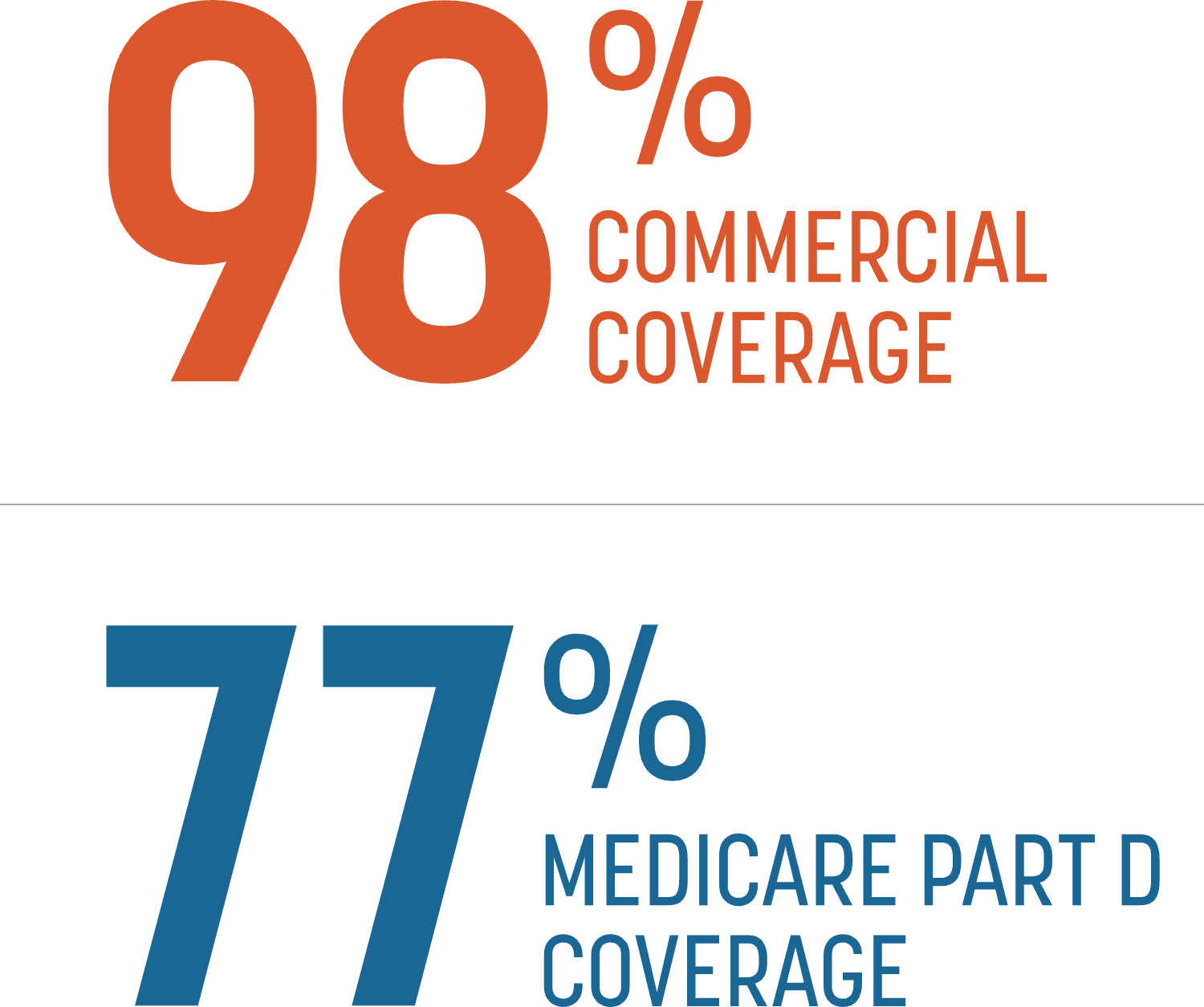
Data courtesy of Managed Markets Insight & Technology as of January 3, 2024.
What to do if TYMLOS is not covered under your patient’s insurance plan?
Advise them to call 1-866-896-5674 to learn about Radius Assist, a patient assistance program available to eligible patients who cannot afford their medication.
Radius Patient Access Support Services
The Support Center will provide you with details related to your patients’ insurance benefits, PA requirements, as well as contact information for SPs.
Call 1-866-896-5674
Patient resources and downloads.

Encourage your patients to register for virtual injection training.
Injection training is provided through our Clinical Educator Network, which helps patients with a prescription understand how to use the pen and self-inject, and supports them as they start and stay on TYMLOS, as directed by you.
The TYMLOS Clinical Educators are also available to provide training for you and your staff on using the TYMLOS pen.
Encourage your patients to register for virtual training at



Patient Brochure
This brochure will help your patients understand osteoporosis as a disease, its associated risk factors, and the role TYMLOS plays in treating it. Inside, patients can find information on the TYMLOS patient support program, directions on storage and use, and how to access a savings card.
Download (opens in a new tab)NEED SUPPORT OR SAMPLES?
Let us know if you want to request samples or a visit from a TYMLOS representative or Field reimbursement manager.
Request Support >